
| Dr. Lawrence J. Albright, Professor Emeritus |
|
Fish and Shellfish Diseases |
|
BSc. (Agr.); Macdonald College/McGill University
M.S., PhD., Oregon State University |
|
|
Office: |
778-782-5626 |
Room B9232 |
|
albright@sfu.ca |
Contact Us |
|
Current Research Program:
We have four main research themes. The first theme deals with the modulation of the non-specific and specific immune systems of cultured aquatic animals, primarily salmonid finfish. We are using natural products to stimulate these systems. Our earlier work has focussed on the use of natural B-1,3 glucans with B-1,6 glucose side chains added in the feed to stimulate the immune systems of salmonids and protect them from natural disease challenges, e.g. furunculosis, under commercial aquaculture conditions. We are currently investigating the use of another natural protein product for stimulating the immune systems of cultured finfish. This product, when added to feed at low concentrations, stimulates the immune system to give protection against bacterial diseases such as furunculosis.
The second theme deals with the use of natural organic products for modulating the growth of cultured salmonids. We are investigating the use of these natural products in feed for enhancing the flesh development of salmonids and suppressing reproductive development.
The third theme deals with anti-phytoplankton therapy of cultured finfish. We are studying the harmful and toxic modes of action of the phyto-plankters, Chaetoceros concavicornis and Heterosigma carterae respectively. Based upon our knowledge of these lethal modes of action, we are developing additives which can be administered to aquacultured finfish by the feed, such that these animals are protected against the harmful and toxic effects of these phytoplankters.
The fourth theme deals with the use of domestic chicken IgY immunoglobulins to protect cultured finfish and crustaceans against disease organisms. We are vaccinating chickens for certain aquatic animal diseases. From these vaccinated chickens the eggs that accumulate IgY immunoglobulins are collected and the yolk contents fed to cultured finfish and crustaceans to protect these aquatic animals from these disease organisms. This enabling technology is useful for protecting aquatic animals from certain diseases for which the aquatic animal cannot readily produce protective antibodies, yet the domestic chicken can do so.
Selected Publications:
Arasteh, N; Aminirissehei, AH; Yousif, A; Albright, LJ; Durance, TD. 2004. Passive immunization of rainbow trout (Oncorhynchus mykiss) with chicken egg yolk immunoglobulins (IgY). AQUACULTURE 231: 23-36.
Lio-Po, GD; Albright, LJ; Traxler, GS; Leano, EM. 2003. Horizontal transmission of epizootic ulcerative syndrome (EUS)-associated virus in the snakehead Ophicephalus striatus under simulated natural conditions. DISEASES OF AQUATIC ORGANISMS 57: 213-220.
Maltby, JB; Albright, LJ; Kennedy, CJ; Higgs, DA. 2003. Effect of route of administration and carrier on bioavailability and kinetics of astaxanthin in Atlantic salmon Salmo salar L.. AQUACULTURE RESEARCH 34: 829-838.
Lio-Po, GD; Albright, LJ; Traxler, GS; Leano, EM. 2001. Pathogenicity of the Epizootic Ulcerative Syndrome (EUS)-associated rhabdovirus to snakehead Ophicephalus striatus. FISH PATHOLOGY 36: 57-66.
Lio-Po, GD; Traxler, GS; Albright, LJ; Leano, EM. 2000. Characterization of a virus obtained from snakeheads Ophicephalus striatus with epizootic ulcerative syndrome (EUS) in the Philippines. DISEASES OF AQUATIC ORGANISMS 43: 191-198.
Lio-Po, GD; Albright, LJ; Michel, C; Leano, EM. 1998. Experimental induction of lesions in snakeheads (Ophicephalus striatus) and catfish (Clarias batrachus) with Aeromonas hydrophila, Aquaspirillum sp., Pseudomonas sp. and Streptococcus sp.. JOURNAL OF APPLIED ICHTHYOLOGY 14: 75-79
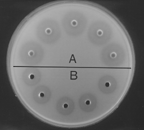 Demonstration of lysozyme activity in whole egg homogenate (A), compared with that of twice-crystallized hen's egg white lysozyme (700 ug/mL) (B). Plate assay (lysoplate) method, with heat-killed M. lysodeikticus incorporated in phosphate-buffered (pH 6.0) agarose gel. Samples (20 uL) were placed in wells, 3.5 mm in diameter and 4 mm deep, and the plate was photographed after 24 h at 20-22C. Dark zones around sample wells are zones of clearing due to bacterial lysis, and are proportional to the concentration of lysozyme.
Demonstration of lysozyme activity in whole egg homogenate (A), compared with that of twice-crystallized hen's egg white lysozyme (700 ug/mL) (B). Plate assay (lysoplate) method, with heat-killed M. lysodeikticus incorporated in phosphate-buffered (pH 6.0) agarose gel. Samples (20 uL) were placed in wells, 3.5 mm in diameter and 4 mm deep, and the plate was photographed after 24 h at 20-22C. Dark zones around sample wells are zones of clearing due to bacterial lysis, and are proportional to the concentration of lysozyme.
|