The Importance of Blood Feeding Arthropods on World Health
 Blood feeders occupy a unique position in world health and disease transmission. Of the ten worst tropical diseases listed by the World Health Organization, 7 are transmitted by biting arthropods (WHO, 1999). Malaria alone affects 300-500 million people annually and of them kills 2 million, mostly children. Other mosquito transmitted diseases include Lymphatic Filariasis (elephantiasis), affecting 120 million people, and Dengue Haemorrhagic Fever (DHF) affecting 20-30 million people annually. Other vector diseases like the hemipteran transmitted Chagas' disease affects the hearts, nervous systems and digestive tracts of 16 to 18 million South Americans, while sleeping sickness (trypanosomiasis), transmitted by the tsetse, Glossina spp. accounts for about 300,000 new cases a year in Africa. Economically, the losses in productivity are huge; in South America annual costs associated with Chagas' disease alone are estimated to be over US$8.5 billion, equivalent to 2.9 million jobs.
Blood feeders occupy a unique position in world health and disease transmission. Of the ten worst tropical diseases listed by the World Health Organization, 7 are transmitted by biting arthropods (WHO, 1999). Malaria alone affects 300-500 million people annually and of them kills 2 million, mostly children. Other mosquito transmitted diseases include Lymphatic Filariasis (elephantiasis), affecting 120 million people, and Dengue Haemorrhagic Fever (DHF) affecting 20-30 million people annually. Other vector diseases like the hemipteran transmitted Chagas' disease affects the hearts, nervous systems and digestive tracts of 16 to 18 million South Americans, while sleeping sickness (trypanosomiasis), transmitted by the tsetse, Glossina spp. accounts for about 300,000 new cases a year in Africa. Economically, the losses in productivity are huge; in South America annual costs associated with Chagas' disease alone are estimated to be over US$8.5 billion, equivalent to 2.9 million jobs.
In recognizing the importance of these vectors, this web site will give an overview of the role saliva plays in blood feeding and parasite transmission. It will focus on the general anti-haemostatic qualities of vector saliva and saliva's role in parasite transmission. The document will limit its scope to three dipterans, (mosquitoes, black flies and phlebotomine sand flies) and one hemipteran group, (the kissing bugs); all short term feeders. For contrast, one long term blood feeding group(the chelicerate hard ticks) will also be considered.
Blood Feeders
Blood feeding arthropods are uniquely suited to act act as vectors for disease and parasite transmission. It is thought that in pre-mammalian times they may have fed on other insects, and because of the similarity and of the concerned flies mouthparts, may have had a common ancestor (AgCan, 1999).
In many cases, arthropods bite to obtain protein for developing eggs, and so often are the female of the species (e.g. most mosquitoes, sand flies and black flies). In other cases, the blood is required for moulting, e.g. the triatome Kissing Bug, Rhodnius prolixus. Ticks require blood for both and so feed once for during each life stage; as a larvae, a nymph, and as an adult. The female dies after she has fed and laid her eggs. Because hemipterans also pass through several moults, and female biting flies lay several batches of eggs during their lives, they will all need to blood feed several times; it is this fact that allows parasites to use the arthropods as intermediate (developmental) hosts, and helps to assure their encountering a final host to complete their own life cycles.
The Role of Saliva in Blood Feeding
Saliva plays several roles in the arthropod. In blood feeders and  non-blood feeders alike, α-amylases and α-glucosidases begin sugar digestion, which is a component of many insect's diet. Saliva can also act as a lubricant that helps maintain delicate feeding mouth parts, and its viscosity may also keep these parts properly association with each other to ensure proper functioning when drawing in blood. In the case of probing arthropods (selenophagic feeders, see below) saliva facilitates locating blood vessels (Ribeiro et al, 1984). Salivary glands can also be important for maintaining body water balance in some long term feeders, like ticks. In their case, water is separated from the meal, moved from the haemocoel into the salivary glands, and pumped back into the host. Hard ticks are obligate blood feeders and, when not blood feeding, don't have the nectar feeding opportunities to take in water that mosquitoes, blackflies and sand flies do. In fact it has been shown that they have hygroscopic saliva with which they cover their palps. At humidities of 60-70% they can absorb all the water they need from the air (Beaty and Marquardt, 1996). Of course saliva also acts the carrier for the pharmacological components that blood feeders require to manage their host's haemostatic response to their feeding. And last but not least, saliva has also become a vehicle for the transportation of parasites from the vector into their final host. How this has happened is of great interest, and has been accomplished by widely differing pathogenic organisms including viruses (Encephalitis), bacteria (Plague), protozoans (African Sleeping Sickness, Malaria) and filarial worms (River Blindness, Chaga's Disease, etc.). Pharmacological components and parasite transmission will be discussed in more detail shortly.
non-blood feeders alike, α-amylases and α-glucosidases begin sugar digestion, which is a component of many insect's diet. Saliva can also act as a lubricant that helps maintain delicate feeding mouth parts, and its viscosity may also keep these parts properly association with each other to ensure proper functioning when drawing in blood. In the case of probing arthropods (selenophagic feeders, see below) saliva facilitates locating blood vessels (Ribeiro et al, 1984). Salivary glands can also be important for maintaining body water balance in some long term feeders, like ticks. In their case, water is separated from the meal, moved from the haemocoel into the salivary glands, and pumped back into the host. Hard ticks are obligate blood feeders and, when not blood feeding, don't have the nectar feeding opportunities to take in water that mosquitoes, blackflies and sand flies do. In fact it has been shown that they have hygroscopic saliva with which they cover their palps. At humidities of 60-70% they can absorb all the water they need from the air (Beaty and Marquardt, 1996). Of course saliva also acts the carrier for the pharmacological components that blood feeders require to manage their host's haemostatic response to their feeding. And last but not least, saliva has also become a vehicle for the transportation of parasites from the vector into their final host. How this has happened is of great interest, and has been accomplished by widely differing pathogenic organisms including viruses (Encephalitis), bacteria (Plague), protozoans (African Sleeping Sickness, Malaria) and filarial worms (River Blindness, Chaga's Disease, etc.). Pharmacological components and parasite transmission will be discussed in more detail shortly.
Problems for Blood Feeders
Generally, arthropods have two methods for locating blood; selenophagic feeding, and telophagic feeding. The former involves inserting needle-like mouthparts into the host's skin with a saw-like motion, and the latter involves lacerating or masticating the skin surface with scissor like mandibles and feeding on the welling blood. While breaching the skin of a host can effectively bypass that first line of defence, it can also ellicit violent reactions that can kill or dislodge the vector. Needless to say, telophagic feeding, practiced by blackflies, sand flies and tsetse, is the more painful method; nevertheless, not every blood feeder has been shown to have pain suppressants. Besides avoiding alerting the host to its physical presence, the blood, once ingested, is also a potentially poisonous meal. Cytolytic and cytotoxic blood compounds can attack the gut cell walls, killing the blood feeder if they are not neutralized.
Even if feeding begins undetected, the actual injury to the host's skin triggers an immediate haemostatic response. The heamostatic response can be broken into two broad processes, the release of platelet aggregation compounds and vasoconstrictors to close very small wounds, followed, if necessary, by inflammation, coagulatiion and an immune response if the wound is more serious or sustained (Ribeiro, 1989). Consequently, short and long term feeders have different anti-haemostatic compounds in their saliva.
Vector Saliva and Host Haemostasis
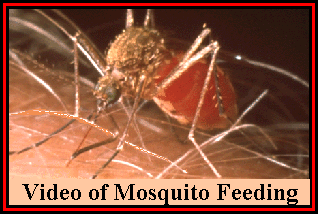 Under normal conditions, victim response to small wounds such as these is very fast and effective. Blood platelets adhere to exposed endothelial collagen and discharge the contents of their granules. This inludes the release of ATP and ADP, which are also released from damaged endothelial cells. ATP and ADP modify the platelet surface causing other platelets to adhere to them. At the same time, arachidoniic acid in the platelet cell membrane, is converted to thromboxane A2 which induces vasoconstriction (Vander et al, 1998). Platelets also contain contractile proteins that tighten to further strengthen the plug. As a result, most small punctures and tears can be sealed in under half a minute of occurring. If damage is more extensive and the wound cannot be closed by the former method, coagulation and immunological reactions follow. It is generally only ticks that have to contend with the latter.
Under normal conditions, victim response to small wounds such as these is very fast and effective. Blood platelets adhere to exposed endothelial collagen and discharge the contents of their granules. This inludes the release of ATP and ADP, which are also released from damaged endothelial cells. ATP and ADP modify the platelet surface causing other platelets to adhere to them. At the same time, arachidoniic acid in the platelet cell membrane, is converted to thromboxane A2 which induces vasoconstriction (Vander et al, 1998). Platelets also contain contractile proteins that tighten to further strengthen the plug. As a result, most small punctures and tears can be sealed in under half a minute of occurring. If damage is more extensive and the wound cannot be closed by the former method, coagulation and immunological reactions follow. It is generally only ticks that have to contend with the latter.
These reactions pose problems for blood feeders who, as a result, have developed an array of pharmacological compounds to defeat the host haemostatic response. As a result, inhibiting platelet aggregation and vasoconstriction are of paramount importance, especially in short term feeders like sand flies, mosquitoes, kissing bugs and black flies. Particularly, an anti-platelet aggregating protein, apyrase, is found in the saliva of all blood feeding arthropods (It is interesting to note that non-blood feeding male mosquitoes have about 20 times less salivary apyrase than the females.) This protein cleaves ATP and ADP to AMP + 2P; and ADP has no platelet aggregating ability. Degranulating platelets also release serotonin, a powerful vasoconstrictor, but because platelet degranulation has been inhibited vasoconstriction is also inhibited. Vasodilators, also very important to short term feeders, increase cell wall permiability (vessel leakage) which contributes to the size of the feeding haemotoma. Vasodilators are not conserved (similar) across the biting arthropods, and so may have originated at later date evolutionarily (Beaty and Marquardt, 1996).
 Longer term feeders, like ticks, have all of these antihaemostatic compounds, but also have sophisticated immuno-suppressives. During the many hours, or days, that a hard tick is attached to its host, its mouthparts are embedded in the skin of its host. This gives mammalian hosts time to marshal coagulation factors and mobilize their sophisticated immunological responses. Ticks, as a result, have much more pharmacologically sophisticated saliva, than short term feeders.
Longer term feeders, like ticks, have all of these antihaemostatic compounds, but also have sophisticated immuno-suppressives. During the many hours, or days, that a hard tick is attached to its host, its mouthparts are embedded in the skin of its host. This gives mammalian hosts time to marshal coagulation factors and mobilize their sophisticated immunological responses. Ticks, as a result, have much more pharmacologically sophisticated saliva, than short term feeders.
Anticoagulation
As mentioned it is thought short term feeders aren't engaged with the host long enough to have to deal with the host's anticoagulation response, but nonetheless, all have anticoagulants in their saliva. Consequently, it is assumed that there is some need for it, possibly to prevent coagulation of blood in the haemotoma or in the feeding tube. (Beaty and Marquardt, 1997). There is a fair amount of variability in the types of anticoagulants employed by blood feeders and most mechanisms and modes of action have not been elucidated. However, they are almost exclusively proteins and most of them have their effect on the shared section of the extrinsic and intrinsic clotting pathways, that is, most act on factor Xa or thrombin, thereby eliminating both coagulation pathways with one compound. There is some information on two of the best known anticoagulants; TAP (tick anticoagulation peptide) and Rhodniin, the latter from Rhodnius prolixus. Both are serine protease inhibitors, but the former cleaves Xa while the latter seems to compete with thrombin for a binding site on fibrinogen, preventing its conversion to fibrin (Beaty and Marquardt, 1997).
Other Tick Salivary Factors
In addition to antiplatelet activity and vasodilators, etc., hard tick saliva includes antihistamines, anticoagulants, immunosuppressives and anti-bradykinins. Their saliva can also destroy anaphylatoxins which stimulate complement plasma proteins to kill bacteria and stimulate the inflammation response, including phagocytosis (Robeiro, 1987;Vander, 1990). However, at the same time, ticks seem to produce a anaphylatoxin-like salivary compound that is similar except for the absence of an terminal arginyl residue. This 'impotent' anaphylatoxin acts as a chemotaxin attracting leucocytes to the feeding site where they stimulate the formation a large haematoma, but cannot stimulate cell degranulation and edema. As a result, the tick feeds from the leukocyte induced haematoma but avoids any further immune response; to add insult to injury, the ticks may even feed on the accumulating neutrophils (Ribeiro, 1987).
Enhanced Parasite Transmission
Not only do vectors evade and manipulate their host's immune system, but a parasite infected vector can also be manipulated by the parasite to enhance the parasite's transmission. This can be done by modifying vector behavior to increase transmission, by modifying vector physiology, for example, by damaging vector salivary contents to reduce apyrase content, or by modifying host systems to improve transmission to a new vector.
When to complexity of parasite life cycles are considered (see Olsen, 1974) along with the astounding ability of blood feeding arthropods to circumvent, and in some cases (ticks) turn heamostatic responses to their advantage, it may not come as too much of a surprise to learn that parasites have come to incorporate the blood feeding process into their transmission cycles. From an evolutionary perspective, there would seem to be a strong selective pressure to use a third party to carry a parasite past the relatively tough and inhospitable integument of the host and be deposited directly into the nourishing blood medium where it can flourish; the original trojan horse strategy.
In the case of selenophagic feeders like mosquitoes parasites enter the host when saliva is injected into the wound to facilitate feeding. Promastigotes of Plasmodium spp are injected in this manner. In the case of telophagic feeders like phlebotamine sand flies, and black flies, the parasites do emerge with the saliva, but because the wound is much more superficial the parasites are mixed into the wound and then move deeper. Triatomine hemipterans inject saliva while they are feeding but parasites are not passed this way. The protozoans of Trypanosoma cruzi emerge from the anus of the kissing bugs as they feed, and are smeared into the wound when the victim scratches the area upon awaking. In the case of onchoserciasis (Onchocerca volvulus)and other fly transmitted filarial worms, the parasite ruptures out of the mouthparts during feeding and enters the wound. In both cases salivary compounds may play a role in the transmission, if not directly, then indirectly, by impeding haemostasis.
Pathogens of long term feedering vectors also seem to take advantage of the advanced immunosuppresive capabilities of its intermediate host to evade the final hosts immune system. For example lyme disease, (Borrelia burgdorferi), transmitted by the hard tick Ixodes scapularis seems immune to the initial phagocytic attack and the host complement system, but only in the presence of host saliva; if injected directly into the final host the virus is quickly destroyed (Ribeiro, 1989).
Other interesting salivary components include those used as chemotaxins. It has been mentioned that hard ticks use anaphylatoxins homologues to attract leucocytes to the wound area but there is also some mystery surrounding how microfilaria infect new mosquitoes when their dermal populations are considered too low to maintain their infectious cycle. It may be that they use mosquito saliva as a signal to congregate at the feeding site and so infect the feeder (Beaty and Marquardt, 1996; Rossignol and Rossignol, 1988).
surrounding how microfilaria infect new mosquitoes when their dermal populations are considered too low to maintain their infectious cycle. It may be that they use mosquito saliva as a signal to congregate at the feeding site and so infect the feeder (Beaty and Marquardt, 1996; Rossignol and Rossignol, 1988).
It can be seen that parasite transmission during, or surrounding, vector feeding has profound effects on the quality of human life. However, the sophistication of the host haemostatic systems involved, and the consequent sophistication of the counter systems evolved by the pathogens, guarantees that the processes involved are incredibly complex and are still in the early to middle stages of being understood. As a result, control of all of the diseases mentioned is still fairly difficult and the area of vector disease biology is a relatively open and exciting field of study.
[ References] [ Links] [ Glossary]
 Blood feeders occupy a unique position in world health and disease transmission. Of the ten worst tropical diseases listed by the World Health Organization, 7 are transmitted by biting arthropods (WHO, 1999). Malaria alone affects 300-500 million people annually and of them kills 2 million, mostly children. Other mosquito transmitted diseases include Lymphatic Filariasis (elephantiasis), affecting 120 million people, and Dengue Haemorrhagic Fever (DHF) affecting 20-30 million people annually. Other vector diseases like the hemipteran transmitted Chagas' disease affects the hearts, nervous systems and digestive tracts of 16 to 18 million South Americans, while sleeping sickness (trypanosomiasis), transmitted by the tsetse, Glossina spp. accounts for about 300,000 new cases a year in Africa. Economically, the losses in productivity are huge; in South America annual costs associated with Chagas' disease alone are estimated to be over US$8.5 billion, equivalent to 2.9 million jobs.
Blood feeders occupy a unique position in world health and disease transmission. Of the ten worst tropical diseases listed by the World Health Organization, 7 are transmitted by biting arthropods (WHO, 1999). Malaria alone affects 300-500 million people annually and of them kills 2 million, mostly children. Other mosquito transmitted diseases include Lymphatic Filariasis (elephantiasis), affecting 120 million people, and Dengue Haemorrhagic Fever (DHF) affecting 20-30 million people annually. Other vector diseases like the hemipteran transmitted Chagas' disease affects the hearts, nervous systems and digestive tracts of 16 to 18 million South Americans, while sleeping sickness (trypanosomiasis), transmitted by the tsetse, Glossina spp. accounts for about 300,000 new cases a year in Africa. Economically, the losses in productivity are huge; in South America annual costs associated with Chagas' disease alone are estimated to be over US$8.5 billion, equivalent to 2.9 million jobs. non-blood feeders alike, α-amylases and α-glucosidases begin sugar digestion, which is a component of many insect's diet. Saliva can also act as a lubricant that helps maintain delicate feeding mouth parts, and its viscosity may also keep these parts properly association with each other to ensure proper functioning when drawing in blood. In the case of probing arthropods (selenophagic feeders, see below) saliva facilitates locating blood vessels (Ribeiro et al, 1984). Salivary glands can also be important for maintaining body water balance in some long term feeders, like ticks. In their case, water is separated from the meal, moved from the haemocoel into the salivary glands, and pumped back into the host. Hard ticks are obligate blood feeders and, when not blood feeding, don't have the nectar feeding opportunities to take in water that mosquitoes, blackflies and sand flies do. In fact it has been shown that they have hygroscopic saliva with which they cover their palps. At humidities of 60-70% they can absorb all the water they need from the air (Beaty and Marquardt, 1996). Of course saliva also acts the carrier for the pharmacological components that blood feeders require to manage their host's haemostatic response to their feeding. And last but not least, saliva has also become a vehicle for the transportation of parasites from the vector into their final host. How this has happened is of great interest, and has been accomplished by widely differing pathogenic organisms including viruses (Encephalitis), bacteria (Plague), protozoans (African Sleeping Sickness, Malaria) and filarial worms (River Blindness, Chaga's Disease, etc.). Pharmacological components and parasite transmission will be discussed in more detail shortly.
non-blood feeders alike, α-amylases and α-glucosidases begin sugar digestion, which is a component of many insect's diet. Saliva can also act as a lubricant that helps maintain delicate feeding mouth parts, and its viscosity may also keep these parts properly association with each other to ensure proper functioning when drawing in blood. In the case of probing arthropods (selenophagic feeders, see below) saliva facilitates locating blood vessels (Ribeiro et al, 1984). Salivary glands can also be important for maintaining body water balance in some long term feeders, like ticks. In their case, water is separated from the meal, moved from the haemocoel into the salivary glands, and pumped back into the host. Hard ticks are obligate blood feeders and, when not blood feeding, don't have the nectar feeding opportunities to take in water that mosquitoes, blackflies and sand flies do. In fact it has been shown that they have hygroscopic saliva with which they cover their palps. At humidities of 60-70% they can absorb all the water they need from the air (Beaty and Marquardt, 1996). Of course saliva also acts the carrier for the pharmacological components that blood feeders require to manage their host's haemostatic response to their feeding. And last but not least, saliva has also become a vehicle for the transportation of parasites from the vector into their final host. How this has happened is of great interest, and has been accomplished by widely differing pathogenic organisms including viruses (Encephalitis), bacteria (Plague), protozoans (African Sleeping Sickness, Malaria) and filarial worms (River Blindness, Chaga's Disease, etc.). Pharmacological components and parasite transmission will be discussed in more detail shortly.
 Longer term feeders, like ticks, have all of these antihaemostatic compounds, but also have sophisticated immuno-suppressives. During the many hours, or days, that a hard tick is attached to its host, its
Longer term feeders, like ticks, have all of these antihaemostatic compounds, but also have sophisticated immuno-suppressives. During the many hours, or days, that a hard tick is attached to its host, its  surrounding how microfilaria infect new mosquitoes when their dermal populations are considered too low to maintain their infectious cycle. It may be that they use mosquito saliva as a signal to congregate at the feeding site and so infect the feeder (Beaty and Marquardt, 1996; Rossignol and Rossignol, 1988).
surrounding how microfilaria infect new mosquitoes when their dermal populations are considered too low to maintain their infectious cycle. It may be that they use mosquito saliva as a signal to congregate at the feeding site and so infect the feeder (Beaty and Marquardt, 1996; Rossignol and Rossignol, 1988).