|
||||||||||||

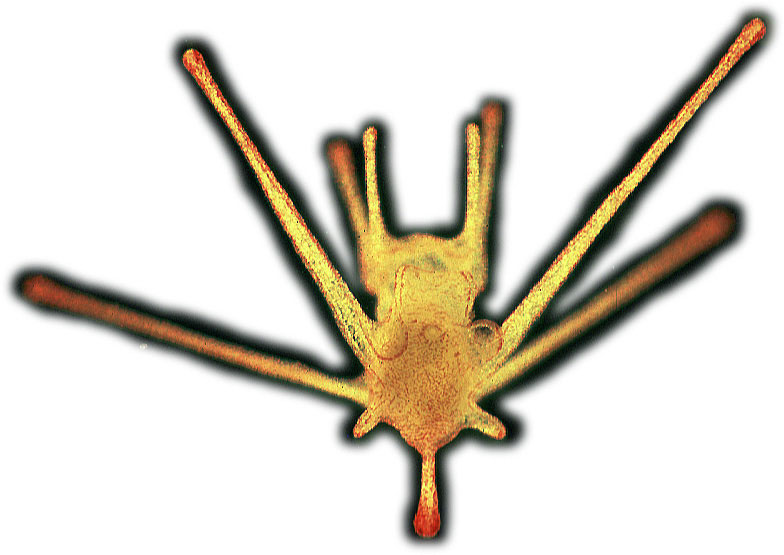
Dr. Michael Hart, Associate Professor, Marine Population Biology Background An enormous variety of marine animals spend the early parts of their lives as specialized planktonic larvae “…in an unregulated state of nature [that] is solitary, poor, nasty, brutish, and short.” (Thomas Hobbes, Leviathan). The general goal of my work is to understand (1) why they do so, and (2) the consequences of doing something else. In many species, adults engage in high-risk sexual behaviour by dumping their gametes into the plankton where (with luck) sperm meets egg to make zygotes. Fertilization rate is often very low, and mortality of offspring is usually very high, so females make very large numbers of tiny, cheap eggs in the hopes that a few will survive. These eggs develop into tiny, vulnerable larvae that often have distinctive adaptations for swimming, feeding, growing, and fending off predators. In some groups, these larvae are so highly specialized for planktonic life that they do not resemble the adult forms at all (for example, the animal at left is a sea urchin, about 1 mm in length, a week or so before metamorphosis into the adult form on the right).These larvae must grow (because initial egg size is much smaller than the size of juvenile stages) and growth is slow (because food particles are often very dilute in the ocean), so the planktonic stage may last weeks or months and result in dispersal and gene flow across large distances (even across ocean basins). In these species, the transition from the larval lifestyle and habitat to the adult involves complex behaviours for selection of a settlement site, followed by a dramatic metamorphosis. In many respects, these life histories are similar to wind-pollinated grasses (with aerial pollen and tiny seeds) and to insects or amphibians (with distinctive larval and adult stages).
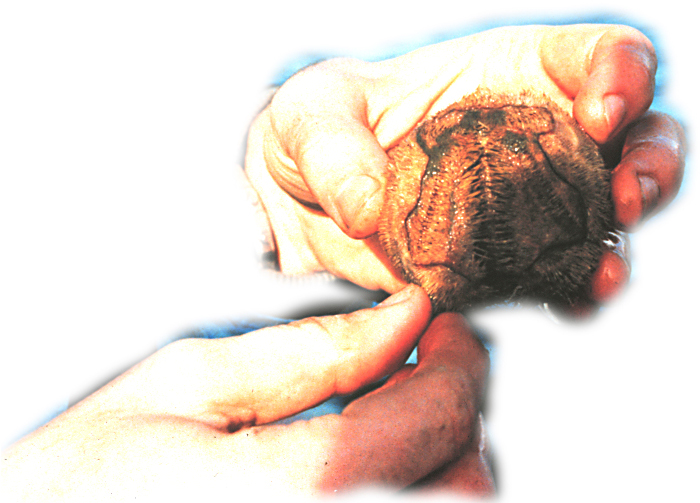
In many respects, these alternative life histories resemble terrestrial vertebrates (with development in egg shells, wombs, or pouches) and animal-pollinated plants (with direct pollen transfer). My lab group studies the population genetics of reproductive variation, mainly in echinoderms. This work uses knowledge of reproductive traits (either measured by us or by others) set in the context of known phylogenetic relationships and ideas about the direction and pattern of evolutionary changes in modes of reproduction. We’re interested in understanding the population genetic effects of these evolutionary changes in mating system, egg size/number tradeoffs, dispersal ability, and larval morphology. Recent work [recently graduated students] in the northwest Atlantic looked at microsatellite and mtDNA population structure in sea urchins, sea stars, and clams in the context of Pleistocene glaciations, range expansion, and hybridization. That research strongly suggests that factors other than simple differences in dispersal ability have a strong effect on population genetic variation in species with widely dispersing planktonic larvae, including:
The new work has several directions: (1) comparison of microsatellite and DNA sequence estimates of inbreeding and population structure among species with different larval forms and mating sytems; (2) analysis of selfing and multiple paternity in species with benthic brooding; (3) study of fertilization kinetics and hybridization in species with planktonic gametes; (4) characterization of genes encoding gamete recognition proteins and their sequence variation within and among species with different mating systems. We have started the comparative population genetics analysis with development of a suite of new microsatellite markers. Similar work is also underway for some British and South African species in Mike Bruford’s lab at Cardiff University in Wales. I am actively recruiting new graduate students or postdocs interested in this work, especially projects (3) and (4).  |
||||||||||||
|
|
||||||||||||

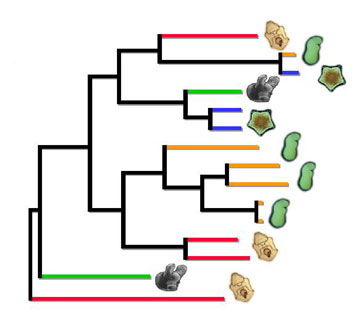 planktonic larval development, but many others have convergently evolved selfing hermaphrodite adults with non-planktonic larval development in egg masses (green) or viviparous brooding with live birth and sibling brood cannibalism (blue).
planktonic larval development, but many others have convergently evolved selfing hermaphrodite adults with non-planktonic larval development in egg masses (green) or viviparous brooding with live birth and sibling brood cannibalism (blue).