 |
 |
C. elegans Developmental Neurobiology Lab |
Home
Research
Publications
Teaching
Lab members
Links
Jobs
Contact
|
|
Introduction |
|
The C. elegans nervous system:
The C. elegans nervous system consists of 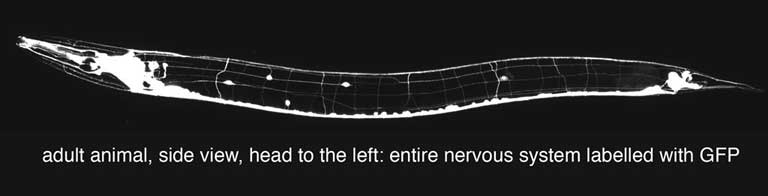 only 302 neurons. Nerve processes (axons and dendrites) have a simple morphology and are frequently unbranched. Reconstructions at the electron microscopic level revealed that the pattern of axonal outgrowth and the synaptic connections among neurons are virtually identical in every animal. Synapses are typically made between neighboring axons within an axon bundle. As a consequence the correct bundling of axons is of considerable importance for the correct wiring of the nervous system and axons have to navigate very precisely even within a bundle in order to find their synaptic targets. only 302 neurons. Nerve processes (axons and dendrites) have a simple morphology and are frequently unbranched. Reconstructions at the electron microscopic level revealed that the pattern of axonal outgrowth and the synaptic connections among neurons are virtually identical in every animal. Synapses are typically made between neighboring axons within an axon bundle. As a consequence the correct bundling of axons is of considerable importance for the correct wiring of the nervous system and axons have to navigate very precisely even within a bundle in order to find their synaptic targets. |
The ventral cord in C. elegans:
The ventral cord is the major axon bundle  that extends along the entire length of the animal. It contains axons from interneurons and motoneurons, which are part of the motor circuit controlling body movement of the animal. The ventral cord consists of two axon bundles, flanking the ventral midline which is populated by motoneuron cell bodies. Almost all the axons run in the right axon bundle and only a few extend in the left bundle. The first neuron to send an axon into the ventral cord - the so called pioneer - is the AVG neuron (Durbin, 1987). It always grows on the right side. Later motoneurons and interneurons send their axons into the ventral cord forming distinct subbundles in the right axon tract. The left ventral cord axon tract is pioneered by one of the PVP axons, which extend into the ventral cord from the posterior end. We can label the different classes of neurons with axons in the ventral cord using cell type specific promoter. Fluorescent proteins like GFP and its derivatives can be used to label different groups of neurons in different color (Hutter, 2004). that extends along the entire length of the animal. It contains axons from interneurons and motoneurons, which are part of the motor circuit controlling body movement of the animal. The ventral cord consists of two axon bundles, flanking the ventral midline which is populated by motoneuron cell bodies. Almost all the axons run in the right axon bundle and only a few extend in the left bundle. The first neuron to send an axon into the ventral cord - the so called pioneer - is the AVG neuron (Durbin, 1987). It always grows on the right side. Later motoneurons and interneurons send their axons into the ventral cord forming distinct subbundles in the right axon tract. The left ventral cord axon tract is pioneered by one of the PVP axons, which extend into the ventral cord from the posterior end. We can label the different classes of neurons with axons in the ventral cord using cell type specific promoter. Fluorescent proteins like GFP and its derivatives can be used to label different groups of neurons in different color (Hutter, 2004). |
Research Topics: |
Pioneer neurons and the navigation of follower axons:
When the pioneer neuron of the left axon  tract (PVPR) is eliminated, the later outgrowing PVQL axon joins the right axon tract (Durbin, 1987). In this case the follower axon strictly depends on the pioneer. However, when the pioneer of the right tract (AVG) is eliminated, later outgrowing axons show only partial navigation defects (Durbin, 1987; Hutter, 2003). In the absence of the pioneer some interneuron as well as motoneuron axons cross the ventral midline and extend in the left axon bundle. Frequently they even cross back and forth between left and right axon tracts, indicating they have partially lost their ability to navigate correctly. Interestingly, the ventral cord asymmetry with most axons on the right side generally does not break down, indicating that the pioneer is not instrumental for this aspect. In genetic screens for mutants affecting ventral cord axon navigation we identified a transcription factor (lin-11), which apparently controls differentiation of the AVG pioneer (Hutter, 2003). In lin-11 mutants AVG fails to express cell type specific markers, indicating that it is not properly differentiating. Interneuron and motoneuron axons show navigation defects very similar to animals, where AVG is eliminated by laser ablation. This transcription factor provides a starting point for the identification of downstream genes controlling pioneer-dependent outgrowth of follower axons. tract (PVPR) is eliminated, the later outgrowing PVQL axon joins the right axon tract (Durbin, 1987). In this case the follower axon strictly depends on the pioneer. However, when the pioneer of the right tract (AVG) is eliminated, later outgrowing axons show only partial navigation defects (Durbin, 1987; Hutter, 2003). In the absence of the pioneer some interneuron as well as motoneuron axons cross the ventral midline and extend in the left axon bundle. Frequently they even cross back and forth between left and right axon tracts, indicating they have partially lost their ability to navigate correctly. Interestingly, the ventral cord asymmetry with most axons on the right side generally does not break down, indicating that the pioneer is not instrumental for this aspect. In genetic screens for mutants affecting ventral cord axon navigation we identified a transcription factor (lin-11), which apparently controls differentiation of the AVG pioneer (Hutter, 2003). In lin-11 mutants AVG fails to express cell type specific markers, indicating that it is not properly differentiating. Interneuron and motoneuron axons show navigation defects very similar to animals, where AVG is eliminated by laser ablation. This transcription factor provides a starting point for the identification of downstream genes controlling pioneer-dependent outgrowth of follower axons. |
Identification of genes controlling axon navigation:
We use different approaches to identify  genes controlling axon navigation. In genetic screens animals are mutagenized, which randomly destroys genes. Progeny with defective axon navigation can be identified easily with GFP markers, which can be used to evaluate axon outgrowth in the living animal. We collected a number of mutants in novel genes with defects ranging from subtle fasciculation defects within the ventral cord to severe defects affecting axon navigation in various parts of the nervous system (Hutter et al., 2005). Several of these mutants were found to be in transcription factors controlling neuronal differentiation (Hutter, 2003; Wacker et al., 2003; Schmid et al., 2006). Apparently the tight transcriptional control of axon guidance genes like receptors for axon guidance signals is an important aspect which is currently is not well understood. Other mutants identified in these screens affect ventral cord asymmetry, pioneer outgrowth itself or the ability of certain followers to use the pioneer for navigation. The detailed characterization of those genes will lead to new insights into the genetic control axon outgrowth. In a complementary approach we use double stranded RNA mediated gene silencing (RNAi) in large scale screens to identify novel axon guidance genes. genes controlling axon navigation. In genetic screens animals are mutagenized, which randomly destroys genes. Progeny with defective axon navigation can be identified easily with GFP markers, which can be used to evaluate axon outgrowth in the living animal. We collected a number of mutants in novel genes with defects ranging from subtle fasciculation defects within the ventral cord to severe defects affecting axon navigation in various parts of the nervous system (Hutter et al., 2005). Several of these mutants were found to be in transcription factors controlling neuronal differentiation (Hutter, 2003; Wacker et al., 2003; Schmid et al., 2006). Apparently the tight transcriptional control of axon guidance genes like receptors for axon guidance signals is an important aspect which is currently is not well understood. Other mutants identified in these screens affect ventral cord asymmetry, pioneer outgrowth itself or the ability of certain followers to use the pioneer for navigation. The detailed characterization of those genes will lead to new insights into the genetic control axon outgrowth. In a complementary approach we use double stranded RNA mediated gene silencing (RNAi) in large scale screens to identify novel axon guidance genes. |
|
|
| |