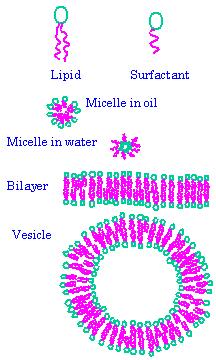
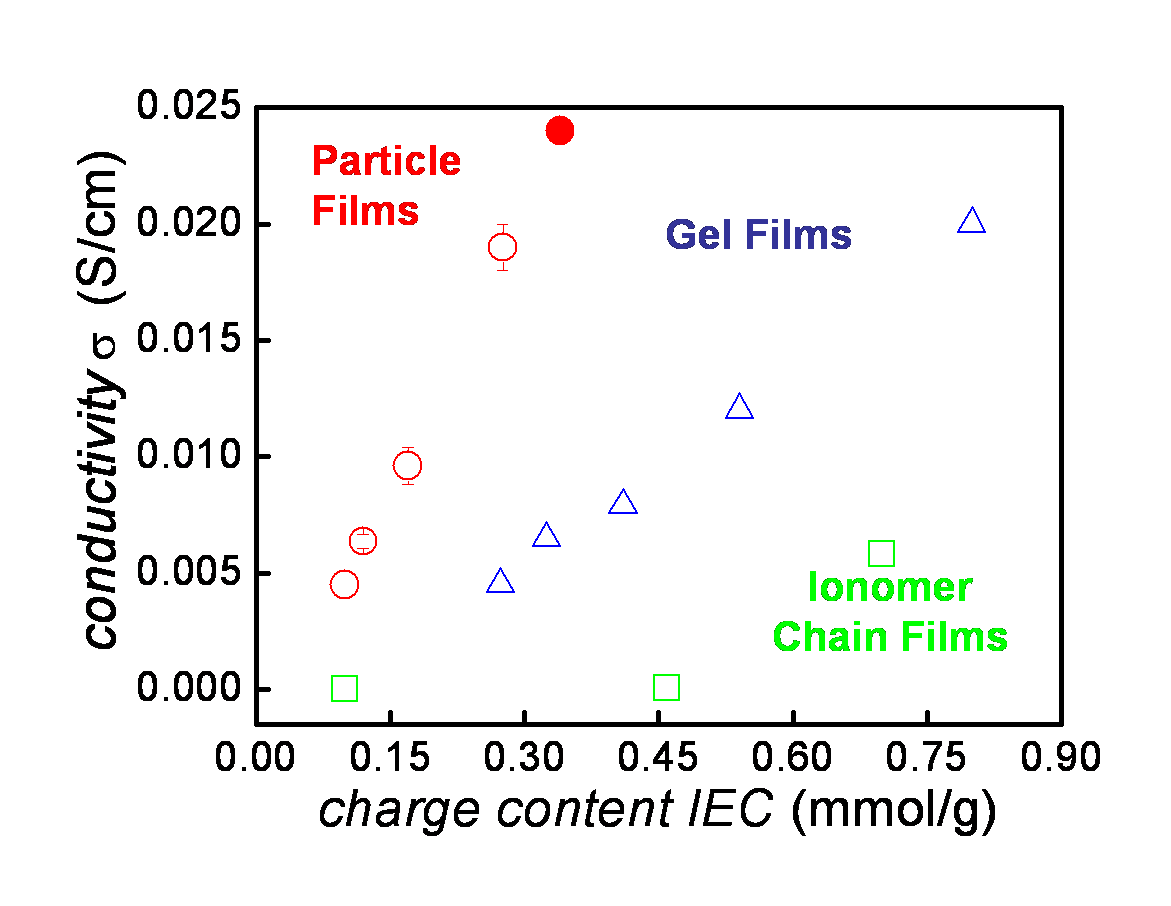
The heart of the polymer electrolyte membrane fuel cell (PEMFC) is an ion-conducting polymer membrane. Good conductivity in PEMFCs depends on polymer morphology and ionic nanostructure. The continuity of the hydrophobic regions is important to keep the membrane from swelling uncontrollably, and the continuity of hydrophilic regions is necessary to provide a continuous path for ion conductivity; ion clustering within the hydrophilic regions is also important. Being able to control this morphology is essential to design of high performance PEMFCs. In collaboration with Steven Holdcroft (Chemistry), we work with model membranes to study the relationship between structure and conductivity. We use Small Angle X-Ray and Small Angle Neutron Scattering to study the structure of these materials.
Anion-conducting Polymer Membranes
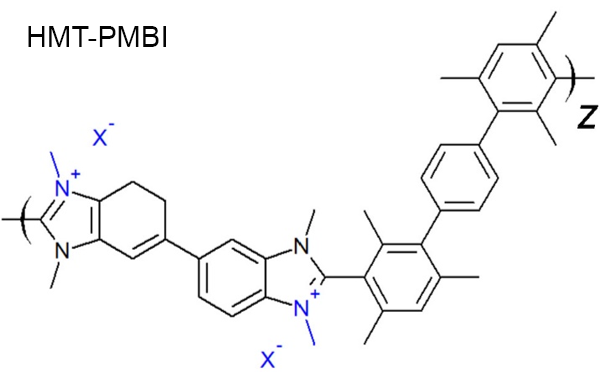
Anion-conducting polymers conduct anions, generally OH-, rather than protons. This type of membrane has advanatages over the standard proton-conducting form. Because the environment is basic rather than acidic, restrictions on catalysts are less severe - platinum is not required! These materials also show improved water management and reduced gas crossover. Our recent projects have focused on studying a family of anion-conducting polymers by medium- and wide-angle X-ray scattering (MAXS and WAXS) combined with molecular dynamics (MD) simulations. The polymers are based on a methylated version of poly(benzimidazole) called hexamethyl-p-terphenylene-poly(methylbenzimidazolium) (HMT-PMBI).
|
|
|
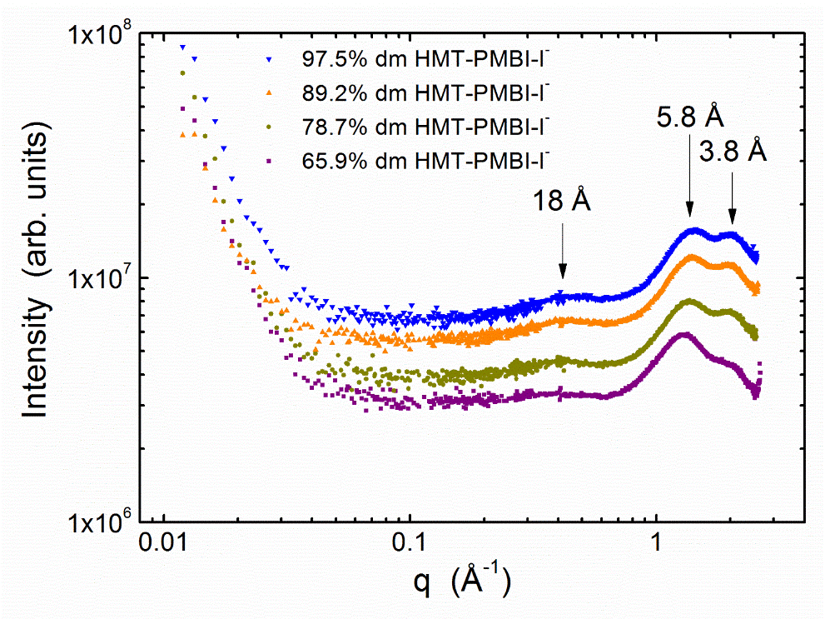
The graph to the right shows MAXS and WAXS data for a series with different charge content. MAXS revealed an interesting lack of both phase separation of hydrophobic and hydrophilic regions and ion-clustering, unusual in good ion-conducting polymers. WAXS revealed an amorphous structure with two main sub-nm length scales. By comparing MD simulations to WAXS data, we attributed the observed scattering features to (1) interchain spacing between the polymer chains and (2) correlation between the positively charged backbone and associated anions. Overall, we have confirmed the interpretation of scattering data by combining MD simulations and scattering experiments.
|
|
|
We have confirmed the interpretation of scattering data through MD simulations. For further details, please see J. Phys. Chem. 122, pp 1730-1737 (2018).
|
|
Graft Copolymer Membranes
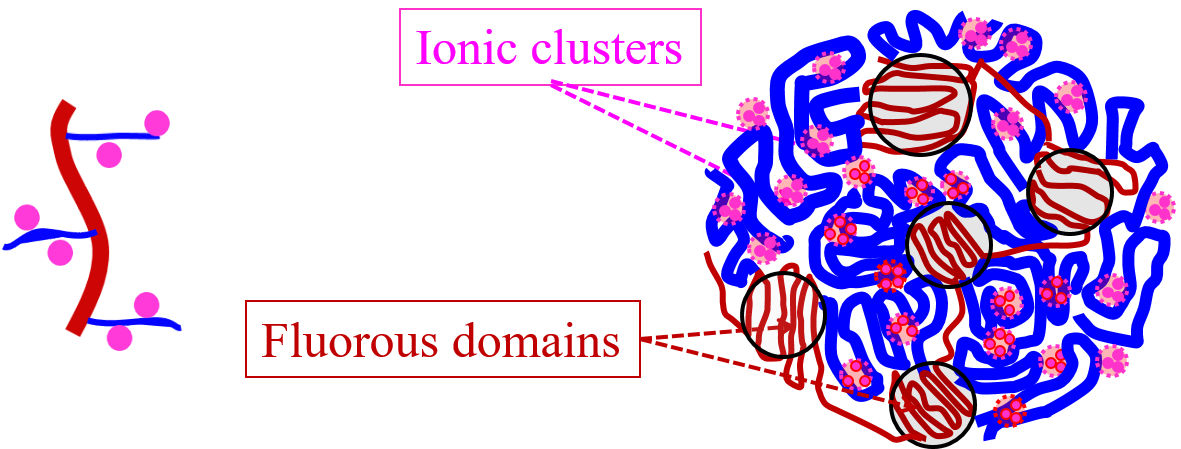
We studied two groups of graft copolymers consisting of a hydrophobic (PVDF) backbone and hydrophilic (sPS) side-chains. The length of the sPS block and its charge content were varied systematically during synthesis. The series of graft copolymers with lower graft density showed some crystallinity and poorer phase separation, consistent with crystallinity impeding phase separation. These samples also had slightly lower conductivity, supporting the view that better phase separation leads to better conductivity.
We concluded that details of the morphology of the fluorous domains, such as their size or organization, do not have a direct impact on swelling or conductivity. But the ability to control water uptake and phase separation through crystallinity of the fluorous domains or the amount of PVDF in the sample has a large impact on the conductivity for a given charge content. The ability to control water uptake through control of molecular architecture is thus a key feature in design of these materials.
|
|
|
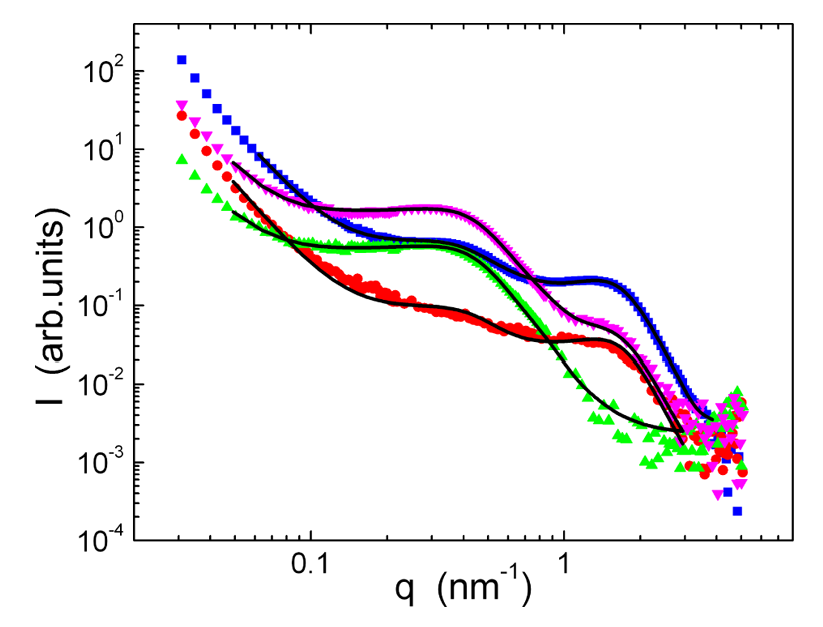
The graph to the right shows SANS scattering spectra for a lower graft density sample in four water mixtures: 100% D2O, 100% H2O, 60% D2O:40% H2O, and 70% D2O:30% H2O. Scattering data revealed a three-phase system consisting of PVDF domains in a matrix consisting of PS-rich and SPS-rich regions.
For further details, please see Macromolecules 46, pp 9676-9687, (2013).
|
|
AB Di-Block Copolymer Membranes
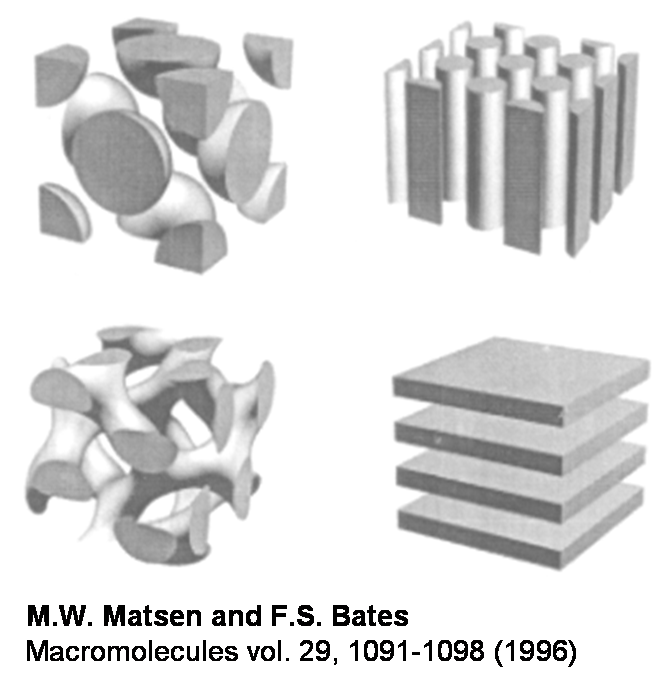
Di-block copolymers are interesting model materials that can be used to study transport properties of polymers. We studied materials made up of a hydrophilic, proton-conducting block (sPS) and a hydrophobic (PVDF) block. In di-block copolymers, different structure can be achieved by varying the relative block sizes, as shown schematically in the figure below.
|
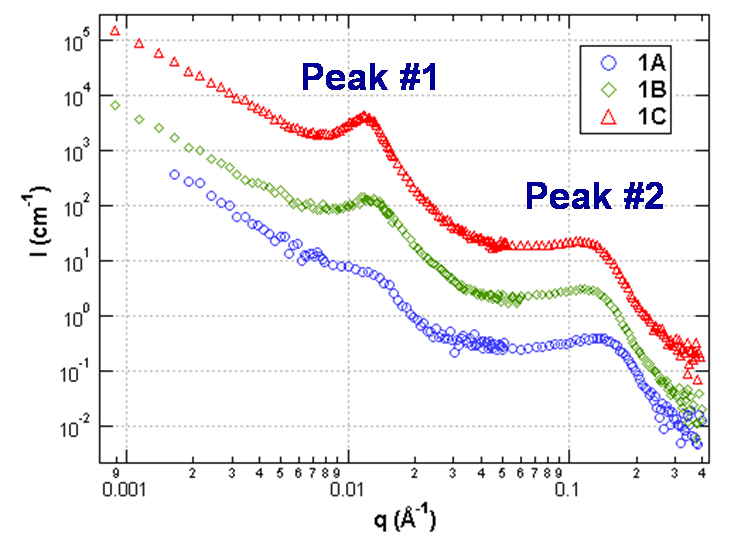
This graph shows scattering spectra for three samples in a series consisting of a poly(vinylidene difluoride-co-hexafluoropropylene) block and a sulfonated polystyrene block where the sulfonation rate is increased from 22% (1A) to 40% (1C). For further details, please see Macromolecules 39, pp 720-730 (2006).
|
|
Return to top
|
|
Phase separation and crystallization play crucial roles in the development of materials including plastics and metal alloys. We are investigating the interplay between these phenomena in colloid-polymer systems where the interaction potential can be precisely tuned by changing the size and concentration of polymer and colloid. In particular, we are studying domain growth and crystallization in colloid-polymer samples that demonstrate gas-crystal and gas-liquid-crystal coexistence. In addition to ground-based studies, we are participating in experiments on the International Space Station. Three samples demonstrating gas-liquid-crystal coexistence were studied as part of BCAT5, a glove-box apparatus installed in the International Space Station.
|

Photo of BCAT 5 apparatus on the International Space Station. The apparatus contains 10 colloidal samples; Samples 6, 7, and 8 are the SFU samples.
|
 Sequence of photos taken of a three-phase colloid-polymer sample as it phase separates in the lab. The latter photos show crystals are forming in the lower phase.
Sequence of photos taken of a three-phase colloid-polymer sample as it phase separates in the lab. The latter photos show crystals are forming in the lower phase.
|
 Sequence of photos taken of a three-phase colloid-polymer as it phase separates on the International Space Station. Phase separation is arrested by formation of crystals within the liquid phase. For further details, please see Phys. Rev. Lett. 109, 195701 (2012).
Sequence of photos taken of a three-phase colloid-polymer as it phase separates on the International Space Station. Phase separation is arrested by formation of crystals within the liquid phase. For further details, please see Phys. Rev. Lett. 109, 195701 (2012).
|
|
Return to top
|