Production of Androgenetic Haploids and Diploids
(Source: G.E. Corley-Smith, C.J. Lim, and B.P. Brandhorst, Simon
Fraser University, B.C., Canada)
Immediately following is the protocol as printed in Eugene's
Zebrafish book in Aug 95. Refinements to protocol
Overview of procedure:
Androgenetic haploids can be produced by holding zebrafish eggs
in coho salmon ovarian fluid, irradiating them with 10,000 R of
x-rays, and then fertilizing them with normal sperm. To produce
diploid androgenotes, the same procedure is followed as for haploid
androgenotes, but is followed by inhibition of the first mitotic
division. The first mitotic division can be inhibited by various
methods. We use heat shock because it is easy to perform and control.
We will keep updates of the protocols on our www zebrafish server.
http://darwin.mbb.sfu.ca/imbb/brandhorst/zfish.html
Equipment Required to Produce Haploid Androgenotes:
1. Irradiation source. If using an x-ray source, it should
produce at least 150 KV. We purchased a Torrex 150D cabinet style
x-ray inspection system (Faxitron X-Ray Corp., Buffalo Grove,
IL., USA. phone: 708 465-9729). Based on research we have performed
using salmon eggs, we believe gamma rays should also work well
for zebrafish eggs.
2. In vitro fertilization supplies. As outlined
in this book under Delayed In Vitro Fertilization Using
Coho Salmon Ovarian Fluid.
Additional Equipment Required to Produce Diploid Androgenotes:
1. Hot water bath. Set to maintain fish water in a beaker
at 28.50.5C. Fish water is a term we apply to the water we use
for raising fish. If desired, embryo water (refer to Recipe Section
of this book) can be used in this protocol, wherever fish
water is indicated. Since timing of the first mitotic division
is temperature dependent, accurate temperature control of this
hot water bath is particularly important for producing diploid
androgenotes.
2. Second hot water bath. Set to maintain fish water in
a beaker at 41.4±0.05C. A calibrated thermometer is required
for this accuracy (e.g. Fisher Scientific, Cat. No. 15041A). Inside
both hot water baths we have beakers containing fish water. To
promote heat transfer, the water in the beakers is constantly
stirred using either a stir paddle or a magnetic stir bar. Temperatures
should be measured in the beakers, since water temperatures in
the beakers are usually slightly lower than in the surrounding
water baths due to thermal loss from evaporation.
3. Heat Shocking Tubes. To transfer eggs between hot water
baths and allow for abrupt thermal changes to be applied to the
eggs, we use uncapped 50 ml polypropylene conical tubes from which
the bottoms have been sliced off and a fine mesh melted on. We
use 153µm pore size Nitex mesh on these heat shocking tubes.
Collection of Eggs:
We collect eggs into approximately 100µl coho ovarian fluid
located in the centre of a 50mm diameter petri dish.
Irradiation of Eggs:
We irradiate eggs at 23 cm from the focal point of the x-ray beam
of the Torrex 150D (shelf 8). Settings used are 145 KV and 5 mA.
X-ray dosimetry indicates that at this distance and electrical
setting, the x-ray output is 12.2 R/sec. Thus, we irradiate for
820 sec to achieve a total dose of 10,000R. Eggs are irradiated
in coho ovarian fluid at room temperature. We attempt to have
a monolayer of eggs with as thin a layer of ovarian fluid over
eggs as possible. The Torrex 150D has a built in 1.2mm beryllium
window. We use no extra filters, as they extend the time required
to deliver 10,000R. If an x-ray machine with sufficient output
is used, a 0.5mm aluminum or copper filter will help to selectively
remove soft x-rays. Soft x-rays (low KeV) are suspected of causing
more cytological damage than hard x-rays which are more selective
in targeting DNA.
Fertilization of Eggs and Production of Haploid Androgenotes:
Proceed as described in Delayed In Vitro Fertilization
using Coho Salmon Ovarian Fluid described in this book.
Embryos that subsequently develop and exhibit the haploid syndrome
are putative haploid androgenotes. No embryos having diploid appearance
should be observed.
Heat shocking to produce diploid androgenotes:
1. Start timer as soon as 0.5 ml of 28.5±0.5C fish water
is added to milt and eggs. This is time = 0.0 minutes.
2. Place petri dish in a 28.5 °C incubator or on a shallow
ledge in a beaker containing 28.5 °C water. After 1 minute,
very gently add 28.5C water to 3/4 fill petri dish.
3. At 5 min, transfer eggs to a heat shocking tube (50ml tube
with net bottom) and suspend tube in beaker containing 28.5C fish
water that is in water bath. Tubes should be suspended, not rested
on bottom of beakers and should be left uncapped.
4. At 13 min, transfer heat shocking tube containing eggs to beaker
containing 41.4C fish water.
5. At 15 min, very gently transfer heat shocking tube containing
eggs back to 28.5C beaker and leave there undisturbed for 1.5
h.
6. After 1.5 h, transfer eggs very gently into petri dishes 3/4
full of water and place in a 28.5C incubator.
7. At 24 h, view developing embryos under dissecting microscope.
At 24 hours, if many haploid and no diploid embryos are observed
in the irradiated and non-heat shocked group, any embryos in the
irradiated and heat shocked group that have a diploid appearance
should be diploid androgenotes.
Assessing Putative Haploid and Diploid Androgenotes:
When attempting to produce diploid androgenotes, it is advantageous
to have at least three groups of eggs: 1) normal diploid control
group; 2) irradiated and not heat shocked (putative haploid androgenotes);
3) irradiated and heat shocked (putative diploid androgenotes).
After collecting eggs, put a small group of eggs aside (control
group) and irradiate the rest. Fertilize all eggs at same time.
Part of the irradiated group can go into 28.5C incubator (potential
haploid androgenotes), and the rest are heat shocked (potential
diploid androgenotes). The control group is to ensure that delayed
in vitro fertilization is working and to allow for the
visual comparison of putative haploid and diploid androgenetic
embryos with normal diploid embryos, as well as for genetic analysis.
Two phenotypes that must be recognizable by the researcher are
the diploid phenotype and the haploid phenotype. Haploid embryos
exhibit what is called the haploid syndrome: shortened body, small
melanocytes. The haploid syndrome can be seen at 24 hours as a
shortened body phenotype (Figure 1). At 48 hours, the shortened
body is easily noticeable and the difference in melanocyte size
starts to become noticeable (Figure 1) and is pronounced by 96
hours (not shown). The development of androgenetic diploid embryos
is initially retarded in relation to diploid control embryos (Figure
1). However, by the end of the first month, the androgenetic diploids
achieve approximately the same size as the diploid control fish.
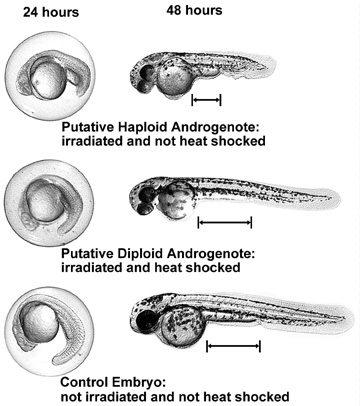 Figure 1: Comparison of haploid and diploid androgenotes and normal
diploid embryos at 24 and 48 hours. Three embryos are shown, each
at two different stages of development. Note: the distance between
the posterior yolk sac margin and the anal pore is greater for
the diploid phenotype than for the haploid phenotype. Click on
image to get higher resolution image (791 KB).
Figure 1: Comparison of haploid and diploid androgenotes and normal
diploid embryos at 24 and 48 hours. Three embryos are shown, each
at two different stages of development. Note: the distance between
the posterior yolk sac margin and the anal pore is greater for
the diploid phenotype than for the haploid phenotype. Click on
image to get higher resolution image (791 KB).
We originally determined the irradiation dosage based on the Hertwig
effect (Hertwig, 1911). To ensure that the irradiation dose is
adequate to destroy the maternal genome in each experiment, we
always include an irradiated and nonheat shocked group. No diploid
phenotypes should ever be observed in this group. If no diploid
phenotypes are observed in the irradiated and non-heat shocked
group, then it is likely that diploid phenotypes in the irradiated
and heat shocked group are androgenetic diploids.
Confirmation of exclusive paternal inheritance requires investigating
the inheritance of parentally polymorphic DNA markers to putative
androgenetic progeny. The lack of homozygous maternally specific
markers in the progeny is strong evidence supporting sole paternal
inheritance, although it does not rule out the possibility of
some maternal leakage.
References:
Hertwig, O. 1911. Die Radiumkrankheit tierischer Keimzellen. Arch.
Mikr. Anat. 77, 1-97.
Die Radiumkrankheit tierischer Keimzellen
means
Die: The
Radiumkrankheit: Radium Disease
tierischer: of animal
Keimzellen Germ Cells
Acknowledgments:
We are indebted to Charline Walker for the *AB line of fish, and
for her extremely helpful advice on in vitro fertilization
and on heat shocking zebrafish eggs.
Updated on August 1995: Tentative results suggest that improved
survival of diploid androgenotes may be achieved by ramping the
heat shock temperature from slightly below the final temperature,
up to the final heat shocking temperature during the first minute
of heat shocking.
Return to List of Protocols
Return to zebrafish home page
 Figure 1: Comparison of haploid and diploid androgenotes and normal
diploid embryos at 24 and 48 hours. Three embryos are shown, each
at two different stages of development. Note: the distance between
the posterior yolk sac margin and the anal pore is greater for
the diploid phenotype than for the haploid phenotype. Click on
image to get higher resolution image (791 KB).
Figure 1: Comparison of haploid and diploid androgenotes and normal
diploid embryos at 24 and 48 hours. Three embryos are shown, each
at two different stages of development. Note: the distance between
the posterior yolk sac margin and the anal pore is greater for
the diploid phenotype than for the haploid phenotype. Click on
image to get higher resolution image (791 KB).