
|
||
Transition-Metal and Lanthanide-Based Supramolecular Coordination PolymersWe are very active in the general synthesis and characterization of coordination polymers for a range of applications. At the heart of these studies is the requirement to understand how to control structure and properties of metal-containing polymers via the design of appropriate ligands and the choice of metals. For example, the ability to control 3-D supramolecular structures is essential to designing materials with useful magnetic, optical, electronic properties or porosity and hence the questions of how to increase structural dimensionality and how to selectively generate coordination polymers with a particular shape or topology are critically important. Developing new magnetic materials also requires an understanding of fundamental magnetic interactions and spin-states. Metallophilic interactions as supramolecular glueGold is a great element! In addition to its economic and romantic value, gold in the +1 oxidation state (d10) has a special property: it forms attractive gold-gold, or aurophilic interactions that can be as strong as hydrogen bonds. As well, compounds with these interactions are often luminescent. We have successfully shown that these aurophilic and related d10-metallophilic interactions could be used as a tool to increase dimensionality in systems containing other metals in addition to the d10 centre. The Leznoff lab concentrates on using metal-cyanides as building blocks for coordination polymers, particularly the unusual linear d10-metal M(CN)2- (M=Au,Ag) units, to take advantage of metallophilic interactions as supramolecular glue; prior to our work there were practically no [Au(CN)2]-based polymers known. For example, we prepared a coordination polymer of the form Cu(tmeda)[Au(CN)2]2 (tmeda = Me2NCH2CH2NMe2). The structure illustrates that aurophilicity is indeed a powerful tool to increase dimensionality, generating a 3-D array from a 1-D polymer (below). We are exploring a wide range of other [M(CN)2]-based polymers with different transition-metals, lanthanides and ancillary ligands, targeting high dimensionality and/or structurally interesting materials with potentially useful applications. 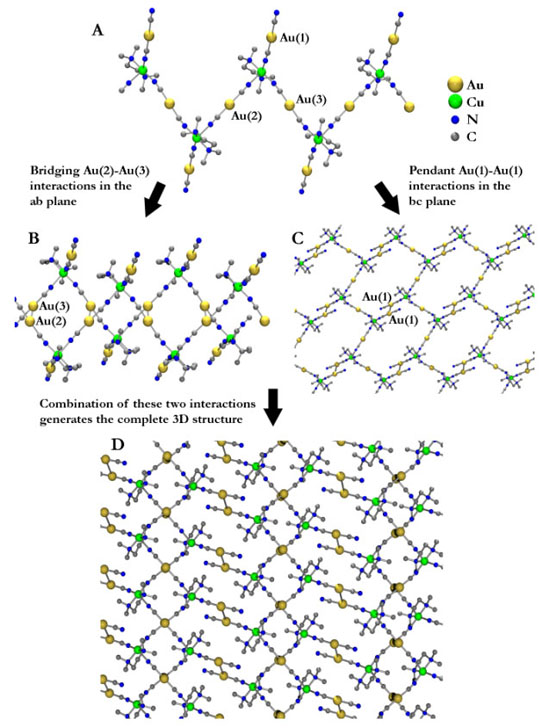 Figure: 3-D structure of Cu(tmeda)[Au(CN)2]2 generated via aurophilic interactions. For more details, see:Leznoff, D.B.; Xue, B-Y; Patrick, B.O.; Sanchez, V.; Thompson, R.C. "An aurophilicity-determined 3-D bimetallic coordination polymer: Using [Au(CN)2]- to increase structural dimensionality through gold•••gold bonds in (tmeda)Cu[Au(CN)2]2.", J. Chem. Soc., Chem. Commun. 2001, 259-260. Download Leznoff, D.B.; Xue, B.-Y.; Batchelor, R.J.; Einstein, F.W.B.; Patrick, B.O. "Gold-gold interactions as crystal engineering design elements in heterometallic coordination polymers", Inorg. Chem., 2001, 40, 6026-34. Download Shorrock, C.J.; Xue, B.-Y.; Kim, P.B.; Batchelor, R.J.; Patrick, B.O.; Leznoff, D.B. "Heterobimetallic coordination polymers incorporating [M(CN)2]- (M=Cu, Ag) and [Ag2(CN)3]- units: Increasing structural dimensionality via M-M' and M•••NC interactions." Inorg. Chem., 2002, 41, 6743-53. Download Molecular magnetic materialsMolecular magnetic materials are a new type of magnetic material composed of molecules rather than metal atoms. This fundamental difference allows us to create types of advanced materials that have 'radically' different properties compared to classical magnets. For example, molecular magnetic materials can be dissolved. They can be highly coloured or transparent. Imagine a luminescent magnet, a chiral magnet or magnetic polymers! These long-term goals have applications in the electronics and computer industries as information storage and display components, as sensors and as molecular switches. As part of a program to investigate magnetic interactions and multi-property materials, we are probing the ability of the luminescent gold and silver cyanide linkers to mediate magnetic exchange. For example, the Cu(tmeda)[Au(CN)2]2 polymer exhibits Au(I)-mediated ferromagnetic interactions (below, left), the first reported example of any magnetic coupling mediated by a diamagnetic Au(I) centre. We are pursuing related ferromagnetically coupled and magnetically ordered materials. 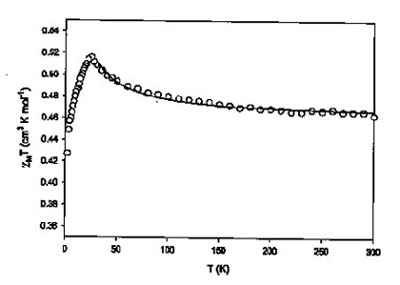 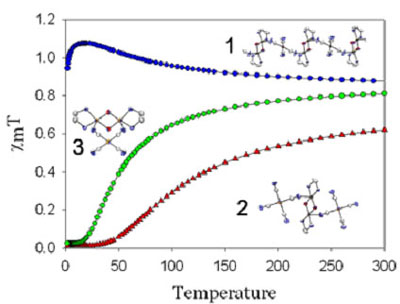 We also recently prepared metal-containing polymers using dinuclear copper(II) cations as building blocks with rarely used square-planar [Au(CN)4]- anions (above, right). Surprisingly, the copper(II) dimers in the product show ferromagnetic or antiferromagnetic coupling, depending on the method of crystallization! We are continuing to explore the structures and properties of [Au(CN)4]- based polymers. For more details, see:Leznoff, D.B.; Xue, B-Y; Patrick, B.O.; Sanchez, V.; Thompson, R.C. "An aurophilicity-determined 3-D bimetallic coordination polymer: Using [Au(CN)2]- to increase structural dimensionality through gold•••gold bonds in (tmeda)Cu[Au(CN)2]2.", J. Chem. Soc., Chem. Commun. 2001, 259-260. Download Shorrock, C.J.; Jong, H.; Batchelor, R.J.; Leznoff, D.B. "[Au(CN)4]- as a supramolecular building block for heterobimetallic coordination polymers" Inorg. Chem., 2003, 42, 3917-3924. Download Leznoff, D.B.; Draper, N.D.; Batchelor, R.J. "Using HgX2 units (X = Cl, CN) to increase structural and magnetic dimensionality in conjunction with (2,2'-bipyridyl)copper(II) building blocks" Polyhedron, 2003, 22, 1735-1743. (Special Issue on Molecular Magnetism, ICCM 2002) Download Katz, M.J.; Shorrock, C.J.; Batchelor, R.J.; Leznoff, D.B. "[Au(CN)4]- as both an intramolecular and intermolecular bidentate ligand with {(tmeda)Cu(μ-OH)}2 dimers: From Antiferro- to Ferromagnetic coupling in Polymorphs", Inorg. Chem., 2006, in press. Vapochromic sensor and porous materialsVapochromic materials, which display optical absorption or luminescence changes upon exposure to vapours of volatile organic compounds, have been a focus of attention due to their potential applications as chemical sensors. We have prepared a series of Cu[Au(CN)2]2(solvent)x coordination polymers that show reversible vapochromic behaviour. When the solid is exposed to vapours of other volatile solvents, significant colour changes result (i.e. the system is vapochromic), indicating that replacement of one solvent for another has occurred. Each solvent can be distinguished easily by the colour of the resulting polymer (from the d-d band of the Cu(II)-centre to which the solvent is bound), and also by the characteristic, sensitive IR νCN signature it generates. Except for the strongest donor solvents and gases, the binding and sensing is completely reversible. As a result, these gold-containing vapochromic sensor materials have potential applications ranging from personal badge monitors or hazardous vapour detection sensors. We are actively pursuing variations and optimizations of this vapochromic coordination polymer system.  We are also exploring porous coordination polymers for a range of sensor and gas storage applications. For example, Cu(pyrazine)[Au(CN)2]2 is a 3-D coordination polymer constructed of Cu[Au(CN)2]2 planes that are connected by pyrazine bridges. However, the 3-D network is not porous, despite the appearance to the contrary, as a second identical network interpenetrates the first to fill the space. The two networks are connected via weak aurophilic interactions, which appear to reinforce the polymer superstructure against thermal decomposition: the decomposition temperatures of the Ni-analogue is 406 °C. 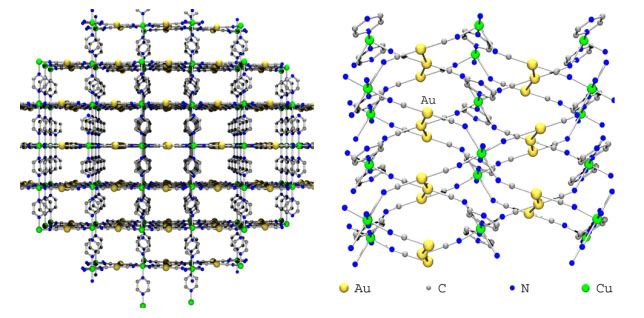 For more details, see:Leznoff, D.B.; Xue, B.-Y.; Stevens, C.L.; Storr, A.; Thompson, R.C.; Patrick, B.O. "Synthesis, structure and magnetic properties of 3-D interpenetrating nets of M(pyrazine)[Au(CN)2]2 (M = Cu, Ni, Co) supported by aurophilic interactions", Polyhedron, 2001, 20, 1247-1254. (Special Issue on Molecular Magnetism, ICCM 2000) Download Lefebvre, J.; Batchelor, R.J.; Leznoff, D.B. "Cu[Au(CN)2]2(DMSO)2: Golden Polymorphs that Exhibit Vapochromic Behaviour" J. Am. Chem. Soc., 2004, 126, 16117-16125. Download Ouyang, L.; Aguiar, P.M.; Batchelor, R.J.; Kroeker, S.; Leznoff, D.B. "A Paramagnetic CuI/CuII/ZnII Coordination Polymer with Multiple CN-Binding Modes and its Solid-State NMR Characterization", Chem. Commun., 2006, in press. Back to the research page. |
||