Research Projects
 |
||
|---|---|---|
The Design and Synthesis of Potent and Selective Alpha Glucosidase Inhibitors |
||
The Salacia reticulata climbing plant has been used for centuries in the treatment of diabetes in Sri Lanka and India. Our group and others have identified many activie glucoside inhibitors found in this plant. These compounds act by inhibiting the break down of starch into smaller sugars, and finally into glucose, which is then absorbed. Similar inhibitors of these same enzymes are already used for treating sufferers of type 2 diabetes as they can reduce blood glucose concentration - a critical component of any treatment of type II diabetes. After our successful structure-proof and synthesis of some of these unusual natural products, we are now designing synthetic methods to produce the next generation analogues, starting from sugars such as glucose and mannose. The biological properties of these novel compounds are then characterized within our group and in collaboration with other researchers.
|
||
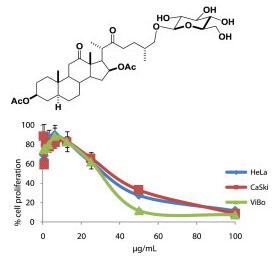
Synthesis and Study of Novel Steroidal Glycosides |
||
This project involves the synthesis of new steroidal glycosides starting from naturally occurring steroidal sapogenins, such as hecogenin and diosgenin. These compounds show interesting properties, including enhanced solubility in polar systems, selective apoptotic anti-cancer activity against cervicouterine cancer cells HeLa, CaSki and ViBo in the micromolar range, and additionally, a null effect on lymphocytes and fibroblasts which implies great selectivity. Once we have synthesized these compounds, we work with our collaborators to evaluate their properties and potential applications.
|
||
 |
||
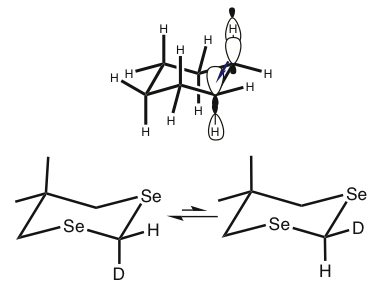
| Exploration of Fundmental Stereoelectronic Effects | ||
We are continuing our ongoing investigations into ubiquitous stereoelectronic phenomena, such as the anomeric effect, by synthesizing and studying novel systems and performing complimentary theoretical simulations. These phenomena are of interest because they play important roles in the structures and reactivites of nearly all organic systems, including carbohydrates, and we aim to increase our understanding of these fundamental effects and elucidate periodic trends. In these studies we employ advanced quantum mechanical calculations, isotopic perturbations, and advanced low-temperature NMR experiments, among other techniques.
|
||
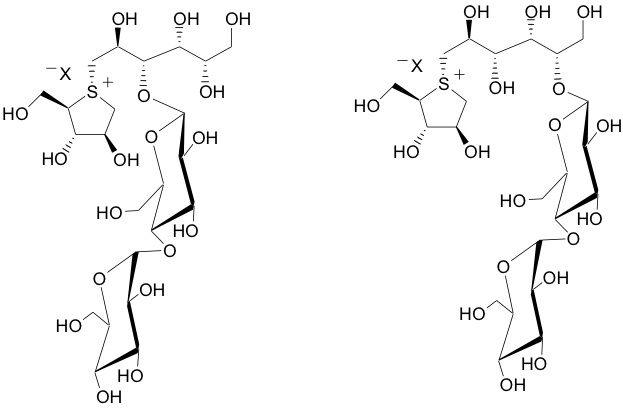
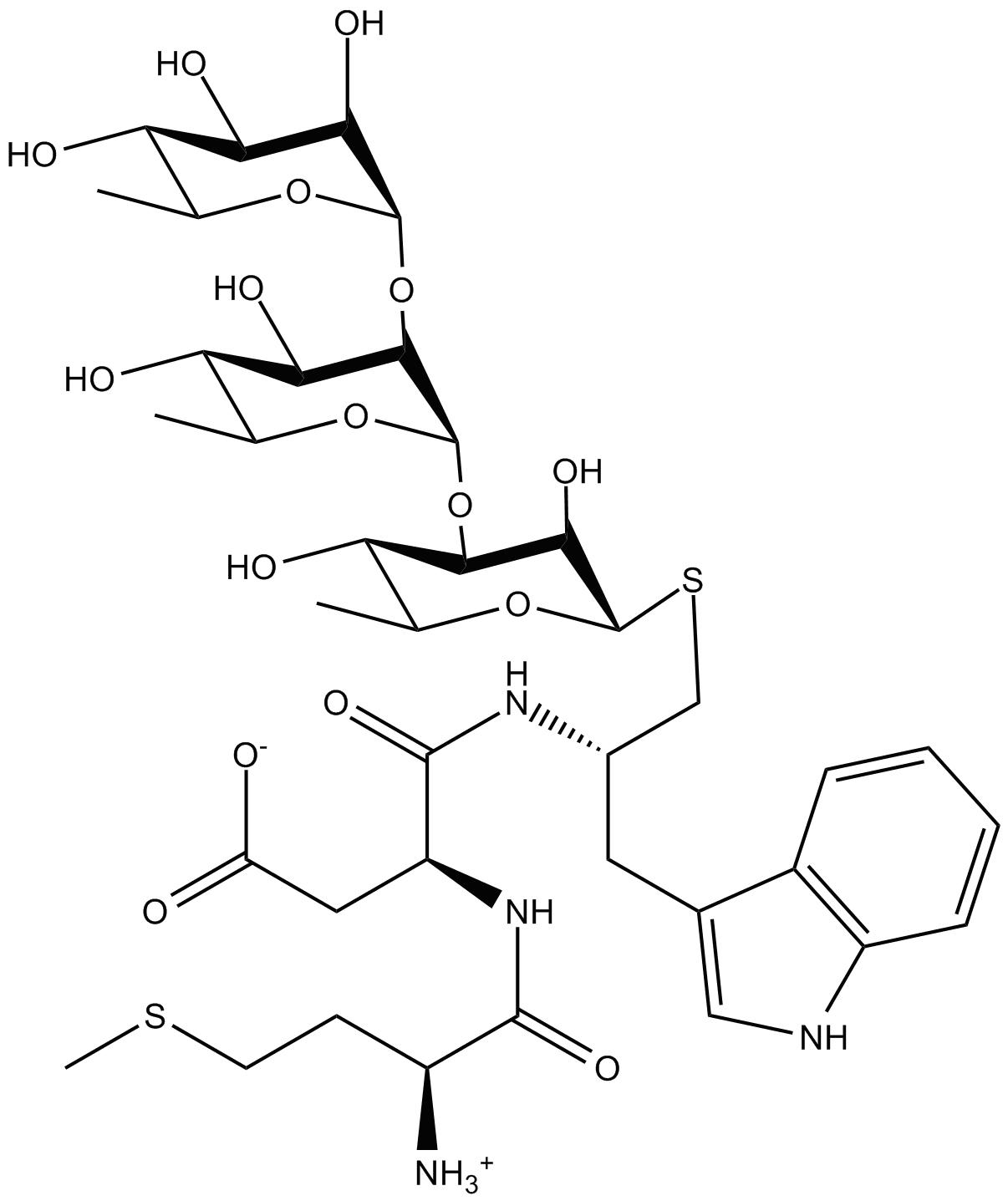
Chimeric Conjugate Vaccine Development |
 |
|
In order to tackle Shigella-caused dysentery (shigellosis), research towards the development of conjugate vaccines against Shigella bacteria is being carried out in our group. The SYA/J6 antibody, a monoclonal antibody raised against Shigella flexneri Y polysaccharide, was used to screen phage-displayed libraries. The screening yielded an octapeptide Met-Asp-Trp-Asn-Met-His-Ala-Ala, which has been used in prime/boost immune strategies by forming a conjugate vaccine with the carrier protein tetanus toxoid (TT). This octapeptide is believed to be a functional mimic of a pentasaccharide, which represents the lipopolysaccharide that coats the cell wall of Shigella flexneri Y. However, the binding modes of the octapeptide and the pentasaccharide are different, and two chimeric glycopeptides were then designed. Chemical synthesis, molecular modeling, and saturation transfer difference NMR are being utilized to investigate the relationship among structure, binding affinity, and immunogenicity.
|
||
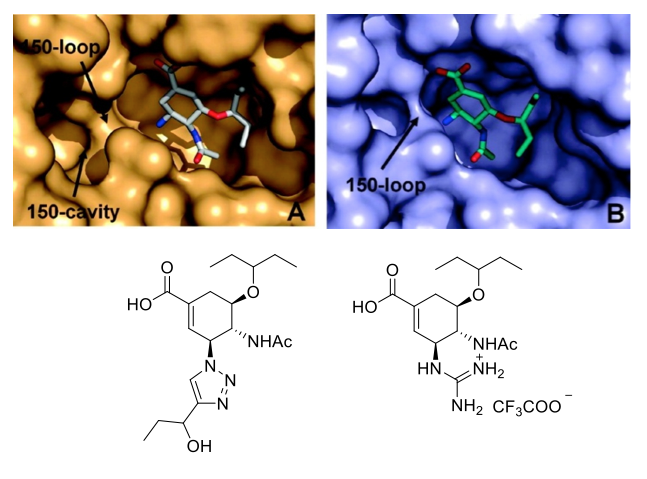 |
Inhibiting Influenza and Overcoming Resistance Influenza is a serious respiratory infectious disease caused by the influenza virus. We are developing a novel class of potential pharmaceuticals for the treatment of influenza infection that inhibit the viral neuraminidase enzyme. While this is a proven drug target, our compounds are designed to better exploit the enzyme's structure. Specifically, we have targeted a nearby cavitiy with the goal of forming additional strong interactions, to create potent inhibitors that are more resistant to viral immunity. Our approach comprises synthesis of next-generation candidate inhibitors, their evaluation in vitro against enzymes from wild-type influenza virus A (IFV-A) and strains that are resistant to Tamiflu, evaluation of selectivity for the viral enzymes over human neuraminidases, and protective ability in mice upon challenge with IFV-A. Ongoing docking and molecular dynamics studies then allow us to understand the nature of the enzyme interactions better and design further compounds for synthesis and evaluation. |
|