
 |
Frederic F. Pio, Associate ProfessorB.Sc., University of Clermont-Fd II, France Phone: (778) 782-5660 |
Research Interest
Our laboratory is focused towards the three dimensional structural characterization of new protein-protein and protein-DNA interaction complexes involved in cancer and apoptosis. Our goal is to identify the critical interactions that modulate affinity and specificity between the different domains. To perform such studies we are using multidisciplinary approaches. They include:
- New protein complex identification using genomic databases, bioinformatics, gene "hunting", computer modeling, and protein-protein interaction assays.
- Minimal interaction domain definition necessary for complex formation using Molecular Biology, Protein Chemistry and Binding Studies.
- Purification of the minimal domain and Reconstitution in vitro of the complex using Protein expression and Purification. Structural characterization of such complexes using biophysics and macromolecular x-ray crystallography.
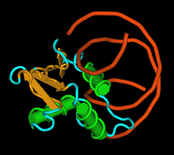 ETS Transcription Factors
ETS Transcription Factors
ETS family of transcription factors are regulatory proteins involved in cancer, cell growth and differentiation. A DNA binding domain of 85 amino acids the ETS domain, which recognizes the ETS DNA binding site core sequence GGAA/T, characterizes them. We have recently solved by x-ray crystallography the first crystal structure of an ETS domain complex to DNA. We are currently investigating the structural analysis of other complexes in this family. Our major interest is to understand how protein-protein interactions mediate protein-DNA specificity and affinity.
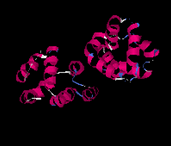 Caspase Recruitment Domain:
Caspase Recruitment Domain:
The caspase recruitment domain or (CARD) of Apaf-1 binds to the CARD of caspase-9 to trigger a proteolytic cascade that leads to apoptotic cell death. The crystal structure of the Apaf-1-caspase-9 complex has been recently solved by x-ray crystallography. Both CARD domains adopt a six-helix bundle fold with Greek key topology surrounding an extensive hydrophobic core. This fold, called the "death Fold", is found in other domains that mediate interactions in apoptotic signaling despite very low sequence identity. So we do not know if Apaf-1-Caspase-9 interaction represent a general mechanism of protein-protein interactions in apoptotic signaling pathways. For this reason we are investigating the identification of new complexes in this family for further structural complex studies.






 Find MBB on Facebook, YouTube
Find MBB on Facebook, YouTube