
Chemistry professor Steven Holdcroft has spent over 30 years researching and developing advanced materials for electrochemical energy conversion and storage, materials that are vital to the creation of sustainable energy conversion technologies such as hydrogen fuel cells and water electrolyzers. Much of his work has to do with “polymers” – otherwise known as plastics – of various compositions. He leads the Holdcroft Research Lab at SFU, where breakthrough discoveries have resulted in new and environmentally friendlier polymers offering clean energy solutions to help combat climate change. His recent paper, On the Evolution of Sulfonated Polyphenylenes as Proton Exchange Membranes describes his work advancing materials for clean energy technologies.
“When we refer to keeping global warming below two degrees by 2050, that’s really the bare minimum to maintain a comfortable atmosphere – now we’re talking about the need to keep it below 1.5 degrees. We are well off both these targets.” says Holdcroft. “Think back to the extreme weather this summer, these spikes in temperature will become the norm. We need to respond urgently. Society needs to adopt technologies that lead to net zero CO2 emissions.”
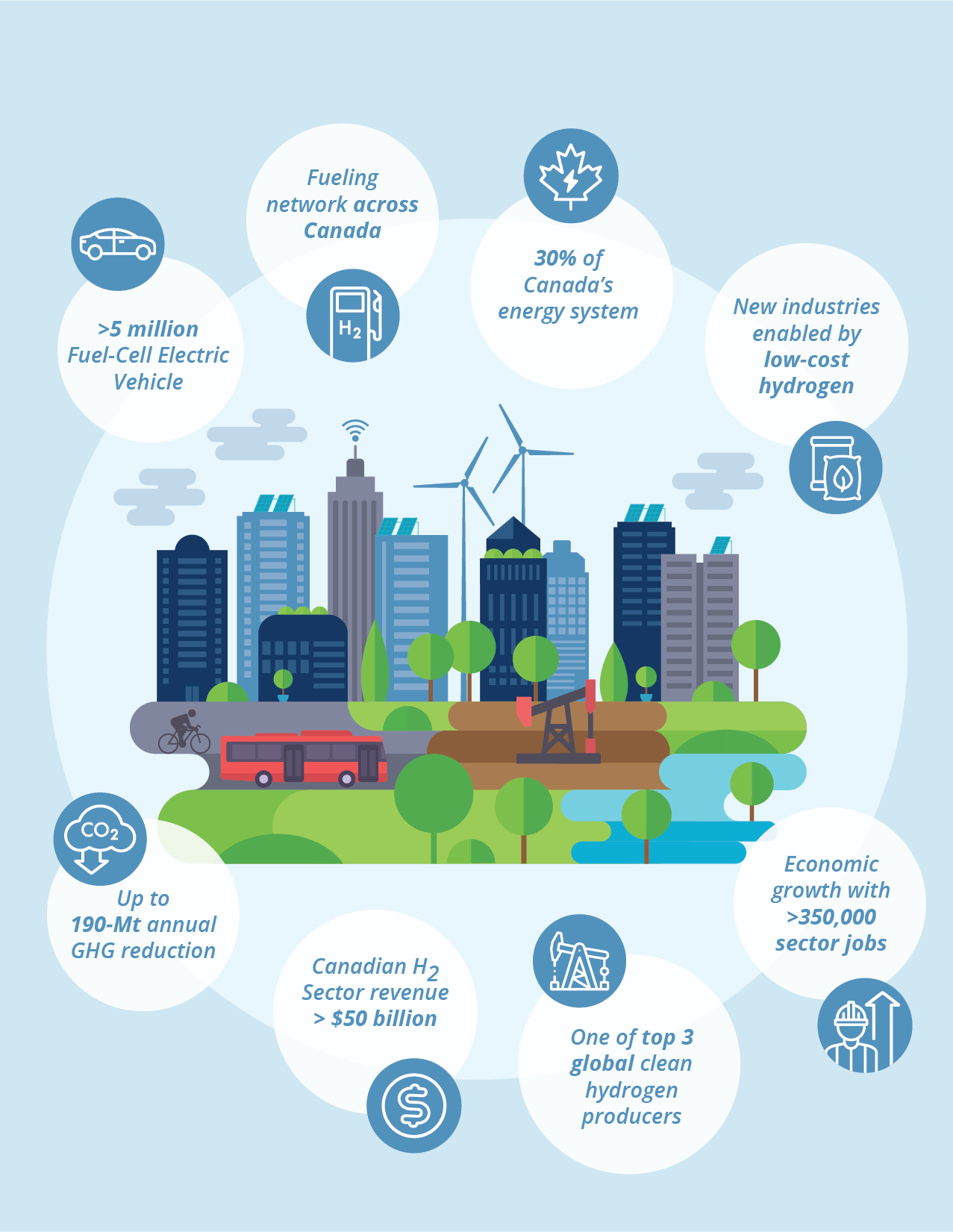
With Canada’s – and the world’s – dependency on fossil fuels how can we hope to achieve net zero? One of the ways to dramatically reduce CO2 emissions is the creation of clean energy from hydrogen. Almost every developed country, including Canada, has adopted strategies that involve moving towards hydrogen energy over the next several decades. Unlike fossil fuel energy, which releases CO2 into the atmosphere, the byproduct of hydrogen energy is water. By 2050, Canada hopes to have more than five million hydrogen fuel cell electric vehicles on the road and deliver 30% of its energy via hydrogen. Currently, countries all over the world are working on similarly ambitious hydrogen strategies, a shift that gives experts like professor Holdcroft hope.
Harnessing the power of clean hydrogen
Much like the energy stored in carbon-based fuels, hydrogen gas stores a large amount of energy that can be converted to electric power. Hydrogen can be produced cleanly from water through a process called electrolysis using renewable energy. The hydrogen can then be stored, transported, and used to power systems as large as a power station and as small as a laptop using fuel cells.
However, there are challenges associated with producing hydrogen inexpensively from water and using it to produce electricity. Electrolyzers need a catalyst and an electrolyte (a solution that conducts ions). In acidic solutions, like battery acid, most cheap catalysts capable of producing hydrogen dissolve, and stable ones are prohibitively expensive, costing more than gold. Electrolytes should preferably be highly caustic (alkaline) so that inexpensive catalysts can be used. Even better, the caustic liquid electrolyte should be replaced by a thin caustic membrane, for an electrolyzer to be scalable and inexpensive.
On the other hand, fuel cells also contain a thin membrane, an acidic one, but to withstand the highly aggressive chemical conditions inside a fuel cell the membrane has to be made from a perfluorinated, Teflon-based material. Perfluorinated plastics and compounds are a growing environmental concern because of their potential toxicity. A non-fluorine based acidic membrane is urgently required as a replacement.
Professor Holdcroft’s lab has been working on both these challenges. Using simple chemical principles, they developed a groundbreaking new class of plastics – sterically protected polybenzimidazoles and polyimidazoles - capable of both transporting hydroxide ions and withstanding highly caustic solutions at high temperature, thus unlocking the future for cheap, clean hydrogen from renewable energy sources. Holdcroft admits that ten years ago he never would have thought it possible for a plastic electrolyte to be sufficiently robust in highly caustic conditions to be used for clean energy technologies.
“If you were to tell me that we would have been able to develop membranes capable of withstanding caustic solutions, to make hydrogen, I never would have believed it – the knowledge just didn’t exist. This discovery and others over the past decade have really opened up the possibilities. The green hydrogen sector is growing leaps and bounds.” says Holdcroft.
From the small gram scale polymer sample created in a test tube, Holdcroft’s group scaled up the polymer to a kilogram, literally what he refers to as “bucket chemistry.” The new polymer membrane was found to have huge potential to meet a market need. It was stable, durable, and could be made affordably in the large quantities (hundreds of kilograms) needed for industrial and consumer use. He and three former students founded Ionomr Innovations Inc., a clean-tech, advanced materials company, to which the technology is licensed from SFU. Under the leadership of experienced managers and directors, the company - now more than 30 strong - is transforming the clean energy sector by enabling high performance hydrogen production from water.

The Holdcroft Lab’s next breakthrough discovery was the creation of a hydrocarbon proton conducting membrane which eliminated the environmental concerns of using fluorine. Not only does the hydrocarbon membrane provide competitive performance to incumbent perfluoro membranes in hydrogen fuel cells, it has the potential to work at a higher temperature and ultimately will cost less. The technology, licensed to Ionomr Innovations, is currently in advanced stages of production.
What’s next? Reclaiming carbon
Holdcroft suggests that even when global hydrogen energy strategies are fully adopted, there still won’t be enough replacement of fossil fuels to fully counter emissions and reduce CO2 to net zero by 2050. An immediate solution to this problem is sequestering carbon from the source, for example from flue gases at refineries, and storing it underground. Moreover, just like the electrolysis of water, CO2 can be electrochemically converted to higher value products like carbon monoxide and alcohols, and even ethylene – a chemical feedstock for making plastics – which is currently refined from fossil fuels. “The possibilities of carbon reclamation are super exciting and the technologies in this area are rapidly developing,” says Holdcroft.
Electrochemical reduction of CO2 needs the right type of membrane that can operate in caustic solutions – technology that that the Holdcroft Lab at SFU is developing – one of the few places in the world with this membrane technology.
“I’m very optimistic about the future because there is path forward, we know the science and technology is available that can make this happen,” says Holdcroft. The materials discoveries today are just the tip of the iceberg, we’ll need to continue to push the boundaries in materials chemistry. To do this and to remain competitive governments need keep investing in Canadian research.”
- Learn more about Steven Holdcroft’s research by watching his President’s Faculty Lecture: Plastics, Basically.
- Professor Holdcroft’s research and the Holdcroft Lab is generously supported by the Natural Sciences and Engineering Research Council of Canada, Innovate BC, Mitacs, Canada Foundation for Innovation, and the Canada Research Chairs Program.
SFU's Scholarly Impact of the Week series does not reflect the opinions or viewpoints of the university, but those of the scholars. The timing of articles in the series is chosen weeks or months in advance, based on a published set of criteria. Any correspondence with university or world events at the time of publication is purely coincidental.
For more information, please see SFU's Code of Faculty Ethics and Responsibilities and the statement on academic freedom.